What is African Trypanosomiasis (Sleeping Sickness)?
African trypanosomiasis (sleeping sickness) is a parasitic disease of people and animals transmitted through vectors of the pathogen. These parasites are protozoa belonging to the genus Trypanosoma, a species Tripanosoma brucei. They are transmitted to humans through the bites of tsetse flies (genus Glossina), which are infected from humans or animals that carry these human pathogenic parasites.
Causes of African Trypanosomiasis (Sleeping Sickness)
There are three morphologically identical subspecies of the causative agent of sleeping sickness: T. brucei brucei — the causative agent of the disease in domestic and wild animals; T. brucei gambiense — the causative agent of the Gambian or West African sleeping sickness of people; . It is endemic in a number of regions of Africa south of the Sahara desert, covering 36 countries with a population of 60 million people. Currently, sleeping sickness infected from 50 to 70 thousand people, and for ~ 2003-2006 g this number has decreased. There are three major epidemics: in 1896-1906, in 1920 and in 1970.
Human infection occurs when the bite of an insect carrier – the tsetse fly (R. Glossina). Usually, human infection by the causative agent of West African trypanosomiasis occurs close to water bodies and along river banks, while infection by the causative agent of East African trypanosomosis occurs in savannas and on the ground in newly cut tropical forests. Man is the main host for T.b. gambiense, and a random host for T.b. rhodesiense, a zoonosis primarily affecting livestock and wild animals. Parasites are morphologically identical: flat, oblong-fusiform in shape, from 12 to 35 μm in length and 1.5–3.5 μm in width. Are mobile, for moving use the wavy translucent membrane stretching along the body. Tripmatostigoitis is usually isolated from the body of an infected person.
Major epidemics of sleeping sickness
Over the past century, several epidemics have occurred in Africa: one between 1896 and 1906, mainly in Uganda and the Congo Basin, one in 1920 in several African countries, and the most recent one began in 1970. The 1920 epidemic was thwarted by the organization of mobile screening by millions of people at risk. By the mid-1960s, this disease had almost disappeared. After this success, surveillance has been weakened, and the disease has reappeared in several areas over the past 30 years. Recent efforts by WHO, national control programs, and nongovernmental organizations have halted and initiated a reversal of the upward trend in new cases of the disease.
Geographical distribution of sleeping sickness
Sleeping sickness threatens millions of people in 36 countries in sub-Saharan Africa. However, only a small proportion of them are under observation with regular check-ups, have access to any medical center capable of providing diagnostic tools, or are protected by means of controlling the carrier of the pathogen.
• In 1986, a group of experts gathered by WHO estimated that about 70 million people live in areas where transmission of the disease may occur.
• In 1998, almost 40,000 cases of the disease were recorded, however, it was considered that this number does not reflect the true situation, and according to an estimate, from 300,000 to 500,000 cases of disease remain undiagnosed and therefore not treated.
• In recent episodes of epidemics in several villages of the Democratic Republic of the Congo (DRC), Angola and South Sudan, the prevalence of the disease has reached 50%. In these communities, sleeping sickness is considered the first or second leading cause of death, even ahead of HIV / AIDS.
• By 2005, surveillance was strengthened, and the number of new cases diagnosed across the continent has decreased significantly; between 1998 and 2004, rates for both forms of the disease, taken together, decreased from 37,991 to 17,616 cases. Currently, the number of cases is estimated to be between 50,000 and 70,000.
Progress in controlling disease
• In 2000, WHO established a public-private partnership with Aventis Pharma (now Sanofi-Aventis), which allowed the creation of a surveillance team that supports endemic countries in their fight against the disease patients.
• In 2006, the success achieved in reducing the number of cases of sleeping sickness prompted a number of private actors to support initial efforts towards eliminating the disease as a public health problem.
The situation today in endemic countries
The prevalence of this disease varies between countries, as well as in different parts of a single country. In 2005, serious outbreaks were observed in Angola, the Democratic Republic of the Congo and Sudan. In the Central African Republic, Chad, Congo, Côte d’Ivoire, Guinea, Malawi, Uganda and the United Republic of Tanzania, sleeping sickness remains a serious public health problem. Countries such as Burkina Faso, Cameroon, Equatorial Guinea, Gabon, Kenya, Mozambique, Nigeria, Rwanda, Zambia and Zimbabwe report less than 50 new cases per year. In countries such as Benin, Botswana, Burundi, Ethiopia, the Gambia, Ghana, Guinea-Bissau, Liberia, Mali, Namibia, Niger, Senegal, Sierra Leone, Swaziland and Togo, transmission apparently ceased, and for several decades no new cases of disease have been reported. However, due to the lack of surveillance and diagnostic specialists, assessing the current situation in a number of endemic countries is difficult.
Pathogenesis during African Trypanosomiasis (Sleeping Sickness)
After inoculation of the parasite, a local inflammatory reaction leads to the formation of an itchy, painful chancre (T. rhodesiense) and regional lymphadenopathy (T. rhodesiense and T. gambiense). Subsequently, when the parasite penetrates the blood and lymphoreticular system, an early hemolymphatic stage of the disease develops. The late stage of the disease develops due to the penetration of the parasite into the central nervous system, where meningoencephalitis develops. The immune response to an infection is characterized, first of all, by the production of specific IgM antibodies, which at an early stage can control parasitemia. However, due to the high variability of trypanosomal surface antigens, the immune response is most often unable to fully cope with the infection. Antigenic variability also explains the recurrent parasitemia characteristic of African trypanosomiasis. Changes in the brain and other organs, primarily in the heart and serous membranes, are characterized by perivascular infiltration of lymphocytes, plasma cells and macrophages. The proliferation of microglia and astrocytes is associated with the severity of neuronal destruction and demyelination.
Symptoms of African Trypanosomiasis (Sleeping Sickness)
The tsetse fly inoculates trypanosomes into the subcutaneous space during bloodsucking. Some parasites penetrate directly into the bloodstream, but most of them remain at the site of inoculation, where they multiply and cause the formation of a local chancre. After the appearance of the chancre, the trypanosomes spread through the interstitial spaces and lymphatic vessels and ultimately enter the blood vessels where they continue to multiply. Parasitemia is low, in the form of waves; each wave disappears when the production of antibodies to the parasite surface glycoprotein antigen increases and appears again after 3–8 days as a new antigen variant is formed. One strain of trypanosomes can form hundreds of antigenic variants, each of which is encoded by a specific gene and is subject to selection under the influence of the host’s antibody response. In the later stages of the disease, parasitemia waves, accompanied by fever and mononuclear leukocytosis, become increasingly rare and irregular. During the dissemination stage, trypanosomes are localized in the small blood vessels of the heart and central nervous system. In the central nervous system, they first cause diffuian leptomeningitis and later perivascular cerebritis. If untreated, this parenchymal inflammation leads to the development of demyelinating panencephalitis. In an experimental infection caused by T. brucei, amastigotic (leishmanial) forms were found, which indicates that there are phases of intracellular tissue development in the life cycle of trypanosomes. This phenomenon may play a role in the latent course of infection.
The mechanism by which trypanosomes cause tissue damage has not been studied. Parasitemia stimulates the production of large quantities of IgM immunoglobulins, possibly in response to the rapid formation of antigenic trypanosome variants. A small proportion of these immunoglobulins are specific protective antibodies; the rest are nonspecific heterophilic antibodies and rheumatoid factor. A high level of immunoglobulins correlates well with the presence of circulating immune complexes, which in turn can activate the kallikrein system and cause vasculitis characteristic of this disease.
Both the Gambian and Rhodesian forms of trypanosomiasis are characterized by the presence of an input lesion or chancre, fever during periods of parasite dissemination, as well as a stage of lesion of the central nervous system. Between these forms of the disease there are some differences in the symptoms, severity and duration of the course; Rhodesian trypanosomiasis is more acute and difficult than the Gambian, and usually ends with the death of the patient during the first year of the disease.
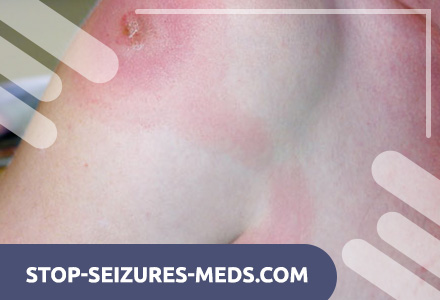
The trypanosomotic chancre appears as an erythematous, painful node at the site of trypanosome inoculation 2–7 days after being bitten by an infected tsetse fly. It can occur anywhere on the body, most often on the head or limbs, and is accompanied by the development of regional lymphadenopathy. Chancre may ulcerate, but ultimately he heals spontaneously. Chancre is more common in Rhodesian trypanosomiasis, possibly due to its more acute course.
After 1 to 2 weeks after infection, as a result of dissemination of parasites, systemic clinical manifestations of the disease become noticeable, however, with Gambian trypanosomiasis, they may appear several years after infection. In typical cases, the patient has a high remitting fever, severe headache, insomnia, impaired ability to concentrate. Developing tachycardia does not match the level of rise in body temperature. Caucasians may have a ring-shaped erythema, resembling marginal erythema. Appear transient dense areas of painful subcutaneous edema, localized on the limbs and in the periorbital region. Characterized by discrete, painless lymphadenopathy with a gradual seal of the lymph nodes and splenomegaly. The nodes in the posterior cervical triangle are especially often enlarged; This is called the symptom of Winterbottom.
In the Gambian trypanosomiasis over a number of years there may be several consecutive exacerbations of the disease with latent periods in between. At the early stage of the disease, the clinical manifestations of the infection are mild, and the disease may remain unrecognized until signs of damage to the central nervous system appear. With rhodesian trypanosomiasis, the fever is more pronounced, depletion develops faster, but the damage to the lymph nodes is less noticeable. Often marked arrhythmia and other signs of myocardial damage; patients usually die from intercurrent infections or myocarditis before the typical sleeping sickness syndrome develops.
The penetration of parasites into the central nervous system can occur in the early period of the disease or be delayed for a period of up to 8 years. Cerebral trypanosomiasis is explosive, causing repeated convulsions or deep coma and death within just a few days. However, in most patients, the disease gradually progresses to the classic picture of sleeping sickness. An absent expression appears on the face, the eyelids are lowered, the lower lip hangs, it becomes more difficult to attract the patient’s attention or to induce him to do something. Patients do not refuse the offered food, but they never ask for it themselves and do not try to make contact with others; their speech gradually becomes blurred and unintelligible. Hand and tongue tremor develops, choreiform movements, seizures with transient paralysis, sphincter incontinence, ophthalmoplegia, pathological plantar reflexes, and finally, against the background of coma, status epilepticus or hyperpyrexia, death inevitably occurs. Death can also occur from intercurrent infections, of which the most important are bacterial and amoebic dysentery, malaria, and also bacterial (often pneumococcal) pneumonia.
Diagnosis of African Trypanosomiasis (Sleeping Sickness)
Diagnosis of the disease and its stage is based on the detection of blood, lymph and cerebrospinal fluid trypanosomes in tests. In the wet preparation, a search for mobile trypanosomes is conducted, in addition, the preparation fixed and colored according to Romanovsky-Giemsa is analyzed. Before microscopic examination, various methods can be applied (differential centrifugation, centrifugation of a layer of leukocytes in microhematocrit tubes, anion-exchange centrifugation, and membrane filtration) to increase the concentration of parasite cells. In order to isolate the causative agent of Rhodesian trypanosomiasis, infection of experimental rats and mice is possible, and in 2-3 days trypanosomes appear in their blood.
Treatment of African Trypanosomiasis (Sleeping Sickness)
Suramin, pentamidine and organic arsenic compounds are traditionally used to treat sleeping sickness. Eflornithine, which has been approved by the FDA as a treatment for the Gambian form of sleeping sickness, is also used. Treatment is chosen depending on the pathogen (Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense), the presence or absence of CNS damage, side effects of drugs and (in some cases) the causative agent’s drug resistance.
At the hemolymphatic stage (no change in CSF) of the Gambian form of sleeping sickness, suramin or eflornithine is prescribed. The drug reserve – pentamidine. At the meningoencephalitic stage (there are changes in the CSF), eflornithine is prescribed.
At the hemolymphatic stage of the Rhodesian form of sleeping sickness, suramin is prescribed, pentamidine serves as a reserve drug. Since suramin and pentamidine pass poorly through the blood-brain barrier, and eflornithine is not always active in relation to Trypanosoma brucei rhodesiense, melarsoprol is prescribed at the meningoencephalitic stage. In case of intolerance to melarsoprol, tryparsamide is prescribed in combination with suramin.
Suramin is highly effective at the hemolymphatic stage of the disease. However, due to the risk of severe side effects, the drug should be administered under the direct supervision of a physician. To exclude anaphylactoid reactions, first administer a test dose (100–200 mg IV). Adults are prescribed 1 g of suramin IV on 1, 3, 7, 14 and 21 days; children – 20 mg / kg (maximum dose – 1 g) / in the same way. A freshly prepared 10% aqueous solution of suramin is used, which is administered by infusion.
In about 1 in 20,000 cases, there is an acute severe reaction to the drug, which can result in death. Nausea, vomiting, hypotension, epileptic seizures develop. In addition, fever, photophobia, pruritus, arthralgia and rash are possible. Of the severe side effects, the nephrotoxic effect is most often noted. During treatment, transient proteinuria is often observed. Before the introduction of each dose it is necessary to conduct a general urine analysis. If proteinuria increases or cylinders and red blood cells appear in the urine sediment, the drug is withdrawn. In renal failure, suramin is contraindicated.
Eflornithine is highly effective in both stages of the Gambian form of sleeping sickness. In clinical trials (on which the FDA recommendations were based), more than 90% of the 600 patients with the meningoencephalitic stage of the disease were cured with its help. The drug is prescribed in a dose of 400 mg / kg / day in / in fractional, every 6 hours for 2 weeks, then – 300 mg / kg / day by mouth for 3-4 weeks. Side effects include diarrhea, anemia, thrombocytopenia, epileptic seizures and hearing loss.
The efficacy of eflornithine for the Rhodesian form of sleeping sickness has not been evaluated.
The disadvantages that prevent the widespread introduction of this drug – high doses and long duration of treatment.
Pentamidine is used as a reserve drug in the hemolymphatic stage of sleeping sickness, although it does not act on some strains of Trypanosoma brucei rhodesiense. For adults and children, the drug is administered intramuscularly or intravenously in a dose of 4 mg / kg / day for 10 days. Acute adverse reactions include nausea, vomiting, tachycardia, and hypotension. As a rule, they are transitory and do not always require discontinuation of the drug. In addition, there may be nephrotoxic effects, changes in the biochemical parameters of liver function, neutropenia, rash, hypoglycemia and aseptic abscesses.
Melarsoprol is the drug of choice at the meningoencephalitic stage of the Rhodesian form of sleeping sickness. Since the drug is effective at both stages of the disease, it is also used at the hemolymphatic stage with the ineffectiveness or intolerance of suramin and pentamidine. However, due to its high toxicity, melarsoprol cannot be considered the drug of choice at the hemolymphatic stage. Adults spend three three-day course of treatment. At the same time, melarsoprol is administered in a dose of 2-3.6 mg / kg / day intravenously fractionally, every 8 hours for 3 days, then, after a break of 1 week, 3.6 mg / kg / day fractionally, every 8 h for 3 days After 10-21 days, the last course of treatment is carried out – the same as the second.
Weakened patients before the treatment with melarsoprol for 2-4 days prescribed suramin. In these cases, it is recommended to start treatment with 18 mg of melarsoprol, gradually increasing the dose to normal. For children, the total dose of the drug should be 18-25 mg / kg; enter it within 1 month. At the same time, they start from 0.36 mg / kg / day IV, gradually increasing the dose at intervals of 1-5 days to a maximum of 3.6 mg / kg / day. A total of 9-10 doses are administered.
Due to the high toxicity of melarsoprol, it is administered very carefully. In some studies, the incidence of drug encephalopathy reached 18%. This pathology is accompanied by high fever, headache, tremors, speech disorders, epileptic seizures, coma; death is possible. At the first signs of encephalopathy, treatment is stopped, but after several days after the symptoms disappear, you can carefully continue the administration of the drug in lower doses.
The ingestion of the drug in the soft tissue is accompanied by a pronounced local reaction. In addition, vomiting, abdominal pain, nephrotoxic and cardiotoxic effects are observed.
Treatment of the meningoencephalitic stage of the Rhodesian form of sleeping sickness in persons who cannot tolerate melarsoprol is difficult. One of the possible approaches is the administration of triparsamide in combination with suramin. However, this combination does not always help, because suramin does not penetrate through the blood-brain barrier, and tryparsamide acts on Trypanosoma brucei rhodesiense much weaker than Trypanosoma brucei gambiense. Tryparsamide is administered at 30 mg / kg (maximum dose – 2 g) in / in 1 time in 5 days – only 12 doses. Suramin is administered at 10 mg / kg i.v. in 1 time per 5 days – also 12 doses. Treatment with tryparsamide may be accompanied by encephalopathy, fever, vomiting, abdominal pain, rash, tinnitus, and visual impairment.
Another approach is to administer eflornithine, however, with respect to Trypanosoma brucei rhodesiense, it is not always active.
Prevention of African Trypanosomiasis (Sleeping Sickness)
Prevention of African trypanosomiasis is to avoid contact with the tsetse fly. Foci of this disease should be visited only when necessary and with the observance of precautionary measures (light clothes – trousers, long-sleeved shirt, overalls for those constantly working in foci, repellents).
Prevents the disease (West African form) for a period of up to 6 months with a single intramuscular administration of pentamidine (lomidine). If necessary, the injection of pentamidine after 6 months is repeated under medical supervision.